
|
|

Hepatitis and Reproduction Deborah L. Smith, MD and Ronald S. Gibbs, MD The risk of viral transmissibility in assisted reproduction is heavily debated. Viral screening policies differ in each country and sometimes between infertility centers in the same country. Medical assistance for procreation in couples where one or both parents are infected with hepatitis raises many concerns about transmission of the infection to the baby, and/or possible contamination of the laboratory technicians, medical staff, and other gametes/embryos that are from virus free parents in the same laboratory. It becomes essential for medical personnel to understand the risk of transmission of hepatitis with assisted reproductive technologies in order to adequately counsel couples who are chronic carriers of viral hepatitis. Viral hepatitis is a term commonly used for several clinically similar, yet etiologically and epidemiologically distinct diseases. Seven human hepatitis viruses have been identified. Hepatitis A, B, C and D are endemic in the United States, Hepatitis E is rarely reported in the United States, Hepatitis F has not been confirmed as a distinct genotype, and Hepatitis G is a newly described flavivirus. This bulletin will review the various viral etiologies of hepatitis, their mode of transmission and implications for infertile couples, pregnant women, and healthcare workers. Hepatitis A Hepatitis A virus (HAV) is an RNA virus and is a major cause of acute hepatitis in the United States (1). The incubation period is 15-50 days and the development of symptoms is inversely related to age. Symptoms include fever, malaise, anorexia, nausea, abdominal discomfort, and jaundice. Hepatitis A virus is primarily transmitted from person-to-person through fecal-oral contamination and is facilitated by poor personal hygiene, poor sanitation, and intimate contact. Most cases of Hepatitis A occur in community-wide outbreaks, with the highest rates of disease in children, adolescents, and young adults. HAV infection is viewed as a rather benign condition. Only 1 in 10,000 patients has a severe or aggressive course (2). Serologic assays are necessary for the diagnosis of HAV because the symptoms associated with HAV infection are not distinguishable from hepatitis caused by other agents. Two serologic assays are commercially available: total antibody to HAV indicates past infection, and immunoglobulin M (IgM) antibody to HAV indicates recent infection, usually within the past 6 months. There is no chronic infection with Hepatitis A, but approximately 15% of persons experience relapsing Hepatitis A, with recurring symptoms lasting as long as 6 months. Healthcare workers are at minimal risk for transmission of HAV (Table 1). Fetal transmission of HAV occurs only with extreme rarity. The incidence of HAV in pregnancy is 1 in 1000 (Table 2) (3). For short-term protection, immune globulin, a preparation of antibodies, is used prophylactically and after exposure to HAV. Hepatitis A vaccine is the best protection and is recommended for persons at high risk for HAV infection (4). HAV vaccination may be given during pregnancy (Table 3). Hepatitis B Hepatitis B, caused by the Hepatitis B virus (HBV), is a well-documented cause of acute and chronic hepatitis. Chronic HBV infection develops in 2% to 6% of adults, 30% to 60% of young children, and as many as 90% of infants (< 1 year) exposed to HBV. The incubation period for acute Hepatitis B is 45-160 days. It usually has an insidious onset, with clinical symptoms similar to those of other types of types of viral hepatitis (2). HBV is of particular interest to the Obstetrician-Gynecologist because of the risk of transmission to the patient, the healthcare providers, and the fetus. Hepatitis B virus is transmitted by parenteral, sexual, or mucosal exposure to infectious body fluids from persons who have either acute or chronic HBV infection. Approximately 25% of regular sexual contacts of HBV infected persons will become seropositive for HBV (Table 4). Healthcare workers, including nurses, are well recognized as being at occupational risk for HBV infection (Table 1). Serologic studies conducted before the implementation of recommendations to prevent transmission of blood-borne pathogens showed that healthcare workers had a prevalence of HBV infection three to five times higher than that of the general population. The risk of transmission from a percutaneous exposure to HBV ranges from 6% to 30% (5). Strict adherence to standard precautions and vaccination with Hepatitis B vaccine, commercially available since 1982, is the most effective way to prevent HBV infection. Hepatitis B vaccine is safe and produces a protective antibody response in 95% of young, healthy adults. The Occupational Safety and Health Administration (OSHA) enacted a rule in 1991 that required employers to provide Hepatitis B vaccine free of charge for all employees at risk of exposure to blood. The most serious health consequence of HBV infection occurs in persons who become chronically infected with HBV. A chronic carrier of HBV is defined as an individual with HBsAg detectable in serum for more than six months. The carrier state occurs equally in both males and females and carriers can transmit the infection to others. HBeAg positivity is associated with a higher rate of transmitting infection in chronic HBsAg carriers (Table 5). These chronically infected persons are not only a major reservoir for transmission of HBV, but also are at risk for serious health consequences. Hepatitis B virus is second only to tobacco as a known human carcinogen. HBV in pregnancy and newborns Acute HBV occurs in one to two of every 1,000 pregnancies, with 1.5% of pregnant women being chronic carriers of HBV. There is no evidence that HBV infection is any more common in pregnancy. The incidence of spontaneous abortion during the first trimester in patients with acute viral hepatitis is increased. Similarly, when viral hepatitis occurs during the third trimester there is an increased incidence of preterm labor. The increased incidence of spontaneous abortion and preterm delivery is probably no higher than that seen with other febrile illnesses (6, 7, 8). Perinatal transmission of HBV occurs quite frequently (Table 2). Transmission can occur as a consequence of intrapartum exposure, transplacental transmission, and breast feeding. Approximately 20-30% of patients who are seropositive for HBsAg will transmit the virus to their neonates in the absence of immunoprophylaxis. In women who are HBsAg and HBeAg positive, the frequency of transmission increases to 90% (9). In addition, the frequency of transmission also depends upon the time during gestation that the infection occurs. If the acute maternal infection occurs in the first trimester, up to 10% of infants will be HBsAg positive. If the mother has an acute infection during the third trimester, 90% of neonates will be positive without prophylaxis. Infants born to mothers who are HBsAg positive should receive both Hepatitis B immune globulin (HBIG) and Hepatitis B vaccine within 12 hours of birth followed by 2 more injections of HBV vaccine in the first 6 months of life (10, 11, 12). The combined use of passive (HBIG) and active immunization is 85-95% effective in preventing neonatal HBV infection (13). Hepatitis C Virus (HCV) More than 50 years ago, it was known that a viral infection different from Hepatitis A and Hepatitis B was responsible for causing hepatitis. In 1974 this agent was given the name Non A Non B hepatitis virus and was found to be responsible for 90% of post blood transfusion hepatitis cases. In 1989, the cause of most cases of the Non A, Non B hepatitis cases were identified and given the designation of Hepatitis C virus (HCV) (14). Hepatitis C is a blood borne virus that is transmitted through percutaneous exposures to blood (transfusions, transplants), needle sticks, or the contamination of supplies shared among hemodialysis patients or IV drug abusers. Hepatitis C virus RNA has also been detected in saliva, urine, breast milk, semen, and menstrual fluid. Therefore, both sexual and vertical transmission have been suggested as alternative modes for transmission of HCV (13, 14, 15, 16, 17, 18, 19). Hepatitis C virus affects more than 1% of the world population (2). It affects 20% of prostitutes, 60% of hemophiliacs, 12% of homosexuals, 30% of sexual partners of HCV infected persons, and 60% of IV drug abusers (15, 16, 17, 18, 19, 20). HCV is the most common cause of post transfusion and chronic viral hepatitis in the Western World and ranks only slightly below chronic alcoholism as the leading cause of cirrhosis and end stage liver disease. HCV can be transmitted vertically to infants making HCV of particular significance to the Obstetrician-Gynecologist. Since most patients (80%) with acute HCV will develop chronic liver disease, [persistently elevated alanine aminotransferase levels (ALT) for more than 6 months after illness onset], it is very important to identify patients at risk. Indications for screening for anti-HCV are listed in Table 6 (2, 20). Currently there are no recommendations regarding restriction of healthcare workers with Hepatitis C. The risk of transmission from an infected healthcare worker to a patient appears to be lower (3%) than the risk of transmitting HBV but higher than the risk of acquiring HIV (21, 22, 23). Newer assays to detect HCV-antibodies have reduced the risk of transmission. Current estimates place the risk of HCV transmission to 1 in 100,000 per blood unit transfused; this risk is higher than the current estimate for the risk of transmission of HIV from a blood transfusion (1 in 450,000) (21, 22, 23). Acute HCV has an incubation period of 14-180 days; 75% of the acute cases are asymptomatic. However, current data suggest 80% of patients infected with HCV will develop chronic liver disease manifested as chronic hepatitis, 35% will develop cirrhosis, and 5% will progress to hepatocellular carcinoma (23, 24). This is in contrast to Hepatitis B in which only 5-10% of patients who are infected with HBV will develop chronic liver disease. Progression of HCV is insidious in most patients. With HCV the average time to clinically significant hepatitis is 10 years after initial exposure, to cirrhosis 20 years, and hepatocellular carcinoma 30 years. Recent evidence suggests that co-infection with HIV modifies the severity of the liver disease and accelerates the disease process. Several tests are available for HCV screening. An enzyme-linked immunosorbent assay (ELISA) was initially used to detect antibody to a single antigen, the c100-3 antigen in the NS-3 region of the HCV (24, 25, 26, 27). The first generation ELISA lacked sensitivity in the early diagnosis of HCV, and had a 50-70% false-positive rate (26). This led to the development of the second and third generation ELISA, which detects three or four antigens from both the structural (c200, c33c, c22-3) and nonstructural regions (NS-5) of the HCV. The sensitivity of these newer tests exceeds 95% and decreases the seronegative "window" to 8 weeks, during which patients with acute Hepatitis C may have a negative test result (26, 27). Because of the problem of nonspecificity of the ELISA, the diagnosis of HCV should be confirmed with a supplemental test. The most widely used method is the recombinant immunosorbent assay (RIBA) in which antibodies are sought to three or four recombinant antigens of HCV. Samples are considered positive if antibodies to two or more of the HCV proteins are present and indeterminate if antibody to only one antigen is found. The most sensitive way to detect HCV RNA is by the polymerase chain reaction (PCR). The presence of viral RNA is the gold standard to make the diagnosis of Hepatitis C infection but this method should not be used as a primary screening test. Ninety percent of patients with a positive RIBA will have a positive HCV-PCR. Quantitative detection of HCV is possible using PCR and is useful for monitoring patients receiving therapy (Table 7). There are very few options for the treatment of HCV. The most widely studied drug for the treatment of HCV is Interferon. Interferon alpha (IFNa) has been used to successfully treat patients with chronic Hepatitis C. However, relapse is common after treatment. Interferons appear to interfere with viral protein synthesis. Interferon for HCV is given at a dose of 3 million units three times a week for six months (10). At this dose, 50% of the treatment groups had a decline in viral load and normalization of ALT levels compared to 9% of controls. However, follow-up studies indicate that there is only a sustained suppression rate of less than 25% at 1 year (27, 28, 29, 30). These studies indicate that patients who normalize their ALT but do not completely clear HCV RNA are more likely to relapse. More recently, combination therapy, INFa plus ribavirin, has resulted in a better response rate compared with monotherapy and is now considered first line therapy (3). 31% of patients who received 24 weeks of combination therapy responded compared to 6% of patients receiving monotherapy for 24 weeks. Both of these drugs are currently contraindicated in pregnancy. However, a review of the literature showed that IFN use in early pregnancy revealed no teratogenic effects in 23 cases. It is believed that the lack of teratogenicity is due to the simple fact that IFN does not cross the placenta. Currently, IFNa is considered a Category C drug and IFN use in pregnancy is not advised. Ribavirin, in contrast, is a Category X drug and should not be used by pregnant women, women attempting pregnancy or their male partners (31, 32). Patients with progressive liver damage who do not respond to medical therapy are candidates for liver transplant. However, 40% of HCV positive patients who get a liver transplant will develop hepatitis within three years of their transplant, making this therapy controversial (27, 28, 30). HCV in Pregnancy and Newborns The seroprevalence of HCV among prenatal populations in the United States has been reported to range from 2.3-4.5% using second generation assays. Among HIV-infected pregnant patients, the seroprevalence rate is as high as 33%. Both of these rates are considerably higher than the rate observed in the general population (1.4%). This stresses the important role the obstetrician-gynecologist plays in the identification and management of women with HCV. Very little is known about the effect of HCV on pregnancy; however, it appears that most women are asymptomatic, and fewer than 10% will have elevated transaminases. Although few women have been studied, there does not appear to be an increase in adverse pregnancy outcome in HCV-infected pregnant women. A correlation between the risk of vertical transmission and maternal viral burden was recently reported by Ohto et al (29). Among all infants born to HIV negative mothers, the risk of HCV transmission was 10%; however, the risk increased to 36% among infants born to women with HCV RNA titers of >106 copies/ml. No woman whose titer was less than 104 copies/ml transmitted the virus to her infant. Similarly, two other studies of HIV-negative mothers have confirmed the strong association between vertical transmission of HCV and maternal viral titers greater than 106 (29). Recent data suggest that the perinatal transmission of HCV is higher in women coinfected with HIV. Based on these results, it was postulated that women with HIV infection who transmitted HCV more frequently had higher maternal HCV viremia. Unfortunately, there is currently no prospective study in HIV seropositive and seronegative women that can definitively answer that question. A cohort of HCV and HIV coinfected women from the Women's and Infants Transmission study showed that the median plasma concentration of HCV RNA was higher among HIV positive women who transmitted HCV to their infants (1.9 x 106 copies/ml) than among those women with HCV-negative infants (3.5 x 105). There was no association between mother-to-infant HCV transmission and gestational age, duration of ruptured membranes, type of delivery, use of fetal scalp electrode, or detection of chorioamnionitis (18, 19, 20, 29, 30). Most infants delivered to HCV infected mothers (>106 copies/ml) progress to chronic hepatitis. The role of breastfeeding in the vertical transmission of HCV has been heavily debated. Hepatitis C virus RNA has been isolated from the colostrum of patients who were viremic, albeit in much smaller quantities (102-104 copies/ml) than was found in their serum (104-108 copies/ml). Even though HCV RNA can be found in breast milk, infection through breastfeeding has not been demonstrated. These findings may be explained by several factors. First, the amount of HCV RNA present in breast milk may be so low as to be unable to infect the newborn. Alternatively, the tiny amount of HCV present in milk may be easily inactivated by gastric juices. Finally, the integrity of the oral and gastrointestinal mucosa may effectively preclude HCV infection by the oral route. Therefore, at the present time, breastfeeding is not contraindicated in patients with Hepatitis C infection by either the CDC or the American Academy of Pediatrics, but this issue is debated amongst experts (19, 20). The physician specializing in obstetrics and gynecology has an integral role in reducing the morbidity and mortality associated with chronic Hepatitis C. Currently routine screening in pregnant women is not recommended. Screening should occur on the basis of high risk factors (Table 5). However, in an infertility practice routine screening for HCV should be offered to couples to help identify asymptomatic women/men who may need therapy before conceiving. Patients should be advised that the course of Hepatitis C may be adversely affected by coinfection with HIV, use of illicit drugs, and the consumption of alcohol. Additionally, Hepatitis A and B vaccination is recommended for all persons with Hepatitis C if they are seronegative for both of these. Specific questions often are asked about the transmissibility of Hepatitis C. Individuals positive for Hepatitis C virus should refrain from donating blood or organs. Safer sexual practices should be strongly encouraged. The physician should recommend that sexual partners of infected patients be tested for HCV antibodies. In households with a member positive for HCV, sharing razors, toothbrushes, and manicuring tools should be avoided. Covering open wounds is recommended. It is not necessary to avoid close contact with family members or to avoid sharing meals or eating utensils. Transmission through intercourse is controversial so the use of condoms is recommended for those not trying to conceive until this issue is clearly resolved. Hepatitis D virus or Delta Hepatitis (HDV) Delta hepatitis (HDV) requires the presence of HBV for replication and expression. Therefore only persons who are infected with HBV will test positive for HDV. Approximately 25% of chronic HBV carriers will be coinfected with HDV. HDV can cause chronic liver disease. Of patients infected with HDV 70-80% will develop cirrhosis and portal hypertension. This is in sharp comparison to those with HBV only where only 15% will develop cirrhosis. Transmission of HDV to the fetus has been documented. Since HBV is needed for HDV replication active measures to prevent transmission of HBV will prevent transmission of HDV. Serum tests for Hepatitis D total antibody and HDV antigen and IgG and IgM antibody are available. HDV antigen will persist in patients with chronic disease (30). Hepatitis E virus (HEV) Hepatitis E is a single stranded RNA virus. HEV is transmitted by the fecal-oral route, with contaminated water being the most common mode of transmission. Hepatitis E is rarely reported in the U.S., but it is endemic in Mexico, Asia, and Africa. HEV is usually self-limited and mild. However, in under developed countries 20% of women infected during their third trimester of pregnancy will die of fulminant hepatitis (30). There is documented transmission of HEV from mother to infant. No Hepatitis E carrier state has been recognized. Serologic tests for HEV are only available in research laboratories. There is no vaccine available. As with HAV, standard precautions will prevent transmission to hospital staff. Sexual contact has not been identified as a risk factor for transmission. Hepatitis F virus (HFV) Hepatitis F has not been confirmed to be a separate genotype and may be a variant of HBV. Hepatitis G virus (HGV or GBV-C) Hepatitis G is a newly described flavivirus that causes chronic infection. In the late 1960's a surgeon with the initials "GB" developed hepatitis. Serum taken from "GB" was passaged serially in primates and was one of the first hepatitis viruses to be transmitted through blood. Subsequent study found GB's hepatitis to be distinct from Hepatitis A, B, C, D, and E. This virus was then called Hepatitis GB or G after the surgeon "GB". HGV is found in 1% of US blood donors and a high proportion of patients with HCV. HGV has been found in 24% of drug users with chronic HCV infection and 47% of patients receiving multiple blood transfusions. There is no data on transmission through sexual contact but a few reported cases have documented vertical transmission. The majority of patients infected with HGV do not get any liver disease. Dual infection with HCV and HGV compared to HCV infection alone does not increase the severity of the disease. More data about GBV/HBV is needed to determine the clinical significance of HGV (30). Viral Hepatitis and assisted reproduction Data are both limited and controversial on the transmission of hepatitis virus during assisted reproduction. Transmission of HBV and HCV are the main areas of research and limited information is now available on HDV and HGV. Concerns over laboratory/nosocomial infection in assisted reproduction clinics has been of great concern since the publication of a case report that described the transmission of HCV from an infected patient undergoing IVF to 2 non-infected patients undergoing IVF within the same clinic during the same time period (33, 34). These 2 cases lead to the legal ban in France from doing ART in HCV positive patients. Additionally, the recent transmission of HBV from HBV contaminated cryopreserved bone marrow samples to HBV negative cryopreserved bone marrow samples has been raised significant concerns for transmission of HBV in cryopreserved semen samples and embryos. Hepatitis and Semen/Embryo Cryopreservation The occurrence of cross-contamination of HBV during liquid nitrogen storage of biological material and subsequent cross-infection of patients has been demonstrated in a few studies (35). Other viruses have also been found to survive direct exposure to liquid nitrogen, including herpes simplex virus, adenovirus (Jones and Darville, 1989), and papillomavirus (Goodman, 1960; Charles and Sir, 1971). There is also evidence to suggest that liquid nitrogen can be contaminated by many micro-organisms, including a wide range of bacterial and fungal species. This information has raised the concern for ART labs of cross-contamination of samples. A recent study reported that the most likely source of contamination was during the cryostorage process, probably from the contaminated liquid nitrogen (35). Thus, there is no doubt that a range of microbes, including Hepatitis B and C, can survive direct exposure to liquid nitrogen, and under certain conditions result in cross-infection. Given the strength of the evidence of liquid nitrogen contamination by microbes and the cross-infection that occurred with bone marrow samples the possibility of contamination or cross-contamination during semen cryopreservation should be taken seriously. Traditionally, semen storage protocols have not involved sterile techniques. Sources of liquid nitrogen contamination include cracking of overfilled straws during freezing, leaking vials or noncapped straws. Other noninfected straws which were inadequately sealed could absorb the contaminated nitrogen, leading to a potential cross-infection during semen processing prior to freezing. The storage of semen in cryovials placed in direct contact with liquid nitrogen also presents a serious risk because a significant proportion of cryovials absorb liquid nitrogen through caps which do not maintain their seal under these conditions. Although the manufacturers usually recommend the use of a second 'skin' called Cryoflex (Product No. 343958; Nunc Nalge International, Roskilde, Denmark) to provide an adequate seal, however, it is common practice to store naked vials in liquid nitrogen. Thus, in some instances, cryovials stored in the liquid phase could absorb potentially contaminated liquid nitrogen. Depending on the length of storage and the exact thawing protocol, any microbes in the absorbed nitrogen may have settled onto the semen interface and be left there when the nitrogen boiled off. Clinical use of this semen, particularly when multiple vials may be inseminated during a treatment cycle, could pose an increased risk of cross-infection. Remember, some diseases such as Hepatitis B require very small amounts of virus to transmit infection. To date, there have been no documented cases of transmission of HBV or HCV in cryopreserved embryos or semen. Consideration of the evidence presented for the transmission of HBV in bone marrow samples leads to the conclusion that cross-infection via the clinical use of cryopreserved semen/embryos is a realistic possibility. This evidence has led to the recent interest in new cryopreservation techniques that have focused on good laboratory practices for the safe storage of gametes and embryos. The main areas of discussion have focused around:
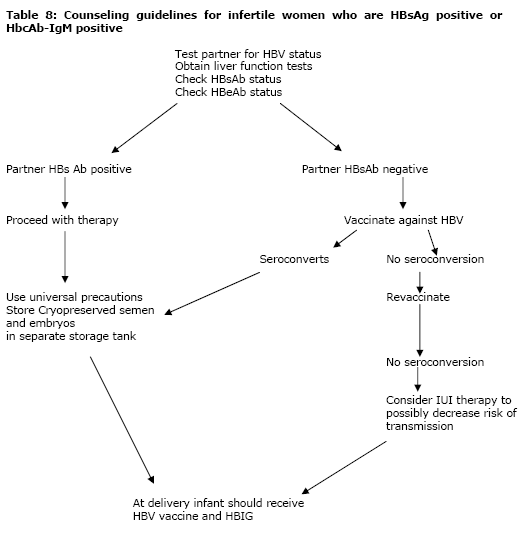
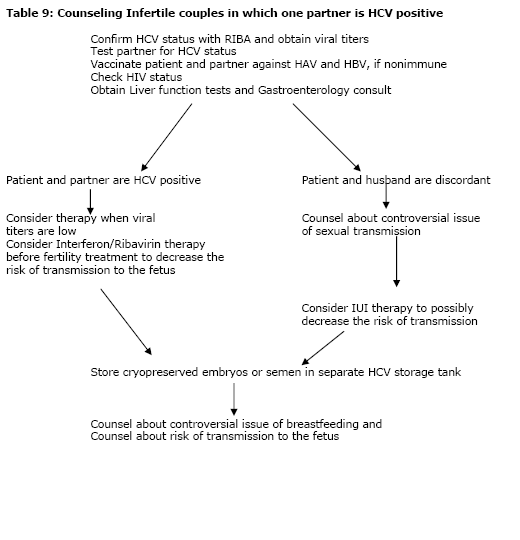
The use of these techniques as possible alternatives to decrease the risk of cross contamination of samples need careful consideration, instead of a knee jerk reaction to mandate immediate change. Issues that should be addressed include safety, cost effectiveness and the effect on pregnancy rates. Currently there are no randomized trials that address all these issues. Until these issues are examined carefully it is difficult to make recommendations for change. Hepatitis and Fresh Insemination Procedures With infertility treatment the risk of exposure to the other partner is only an issue when the couple's hepatitis status is discordant. For couples where one partner is HbsAg positive, the best option is HBV vaccination to prevent transmission. Since 95% of patients will seroconvert after vaccination, we will rarely see a patient in which the patient and partner are at risk of transmission. However, with HCV the risks are different, as no vaccination for HCV is currently available. Methods to decrease the viral load of HCV in the semen sample seems to be an appropriate treatment plan until the issue of sexual transmission of HCV is clarified. Research studies have evaluated methods of semen preparation to decrease the amount of hepatitis virus in the inseminated sample. Information on transmission of HBV and HCV in IUI samples is limited and separation and removal of the infective fraction of the ejaculates has not been well studied. Levy et al, evaluated HCV RNA in sera, semen, and in sperm fraction after Percoll gradient preparation. HCV RNA was detected in 5% (2/39) of the semen samples but 0% of the samples after Percoll selection. This suggests that the risk of transmission is low, but this has never been studied in human subjects. As a comparison, there is also very limited data evaluating HIV transmission in semen. Semen from HIV positive men was prepared by gradient centrifugation, then inseminated in their partner. The absence of maternal seroconversion after more than 1000 intrauterine inseminations using these purified sperm fractions is reassuring that the transmission rates of viruses may be low with IUI. However, this study only evaluated the transmission of HIV-1. This data brings to the forefront the ethical issue of whether a physician has the right to refuse infertility treatment to a well informed couple where one of the partners is HCV-viremic. Even if the occurrence of HCV in semen after Percoll gradient is low, one must consider the ultimate risk of transmission to the mother and/or child. It is also important to consider the risks to the employees preparing the sample, laboratory contamination of other noninfected couples' gametes through cryopreservation and manipulation before deciding to offer care to HCV viremic patients (33, 34, 35, 36). Recommendations for treating infertile couples Testing for HBsAg, HBcAb-IgM, HCV, VDRL, HTLV 1 & 2, and HIV 1 & 2 status should be performed on couples prior to cryopreservation of semen or embryos. Since methods are available (vaccination, possibly IUI) to decrease the risk of transmission to the partner or fetus consideration should be given to testing all couples prior to fertility therapy for these infectious diseases. All hepatitis patients need to be counseled about the risks of transmission to their partner, staff, and their children (Table 1). All office staff should be vaccinated for HBV and universal precautions utilized when handling blood and body fluids for all patients. Until there is a better understanding of the risk of transmission in cryopreserved semen samples and embryos, separate storage tanks for HBV and HCV patients should be established. For specimens with an unknown status, cryopreserved semen should be stored in separate quarantine tanks until the results of infectious disease testing is known. Laboratories should use the company's guidelines for the brands of equipment they utilize for sealing straws and vials to limit leakage of viral agents to their liquid nitrogen storage tanks (Tables 8, 9). For women with HCV viral titers >106, interferon/Ribaviran therapy should be utilized before attempts at pregnancy to decrease the risk of transmission to the fetus. Conclusion Medical assistance for procreation raises many ethical issues with regard to hepatitis transmission. Viral screening and adequate counseling will decrease the risk of transmission of the hepatitis infection to the baby, laboratory/medical staff, and other gametes/embryos in the same lab. Testing infertility patients for HBV and HCV should be offered before starting therapy to decrease the possible risks of transmission of these viral diseases to their sexual partner, to the staff members and to other disease-free embryos/patients in the same laboratory. Summary
Table 1: Risk of transmission to and from health care workers
Table 2: Risk of transmission of hepatitis to the fetus
Table 3: Hepatitis A vaccine is recommended for the following:
Table 4: Risk factors for Hepatitis B (15, 16, 17)
Table 5: Interpretation of serologic testing in patients with HBV infection
Table 6: Indications for screening for HCV (27, 28, 29, 30)
Table 7: Clinical indication for HCV-PCR testing
REFERENCES
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Advanced Reproductive Medicine
Detailed Directions to Our Offices |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||